Looking for help with the class exercise that I am currently stuck on:
Iron isotopes.
The measured δ56Fe for dissolved Fe in the Atlantic Ocean is found in the supplementary data file found here . Assume the following endmember δ56Fe for these main sources, as discussed in class:
δ56Fe dust = +0.68 ‰
δ56Fe sediments = -2.40 ‰
(a) Calculate the fraction of the dissolved Fe in the upper 500 m in the Atlantic Ocean that is from dust or reducing sediments. On average, which is the greater source in this depth range and what percentage of the dissolved Fe does this source account for
Known relationship from the equation 2 of the paper linked above:
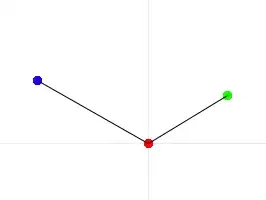
So here Fe1 would be the dust source, Fe2 would be the sediment source, and Fe dissolved is the vector of δ56Fe values in the data (column 8). I began by filtering in R to have a d.f. of samples from 0-500m.