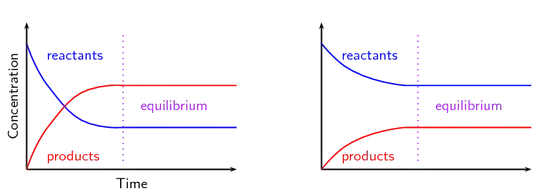
I am try make graphs used scipy and matplotlib, my idea is variable k and concentration in for interactive graphics for equilibrium chemistry. Maybe my equation have error, no make graphics type example bellow, just one example graph finale
import numpy as np
from scipy.integrate import odeint
import matplotlib.pyplot as plt
# To reaction A + B <=> C + D
k1=0.05
k2=0.05
tf= 200
dt=0.2
t = np.arange(0,tf+0.01, dt)
n = len(t)
Ca= np.ones(n)
Ca= np.ones(n)
Cb= np.ones(n)
Cc= np.zeros(n)
Cd= np.zeros(n)
def dC(C,tm):
Ca,Cb,Cc,Cd =C
r1 =k1*Ca
r2 =-k2*Ca
d1 = -r1
d2 = -r1 -r2
d3= r1-r2
d4= r2
return [d1,d2,d3,d4]
C =odeint(dC,[1,1,0,0], t)
Ca= C[:,0]
Cb= C[:,1]
Cc= C[:,0]
Cd= C[:,1]
plt.plot(t,Ca, 'r--', linewidth=2.0)
plt.plot(t,Cb, 'k--', linewidth=2.0)
plt.plot(t,Cc, 'b--', linewidth=2.0)
plt.plot(t,Cd, 'm--', linewidth=2.0)
plt.show()