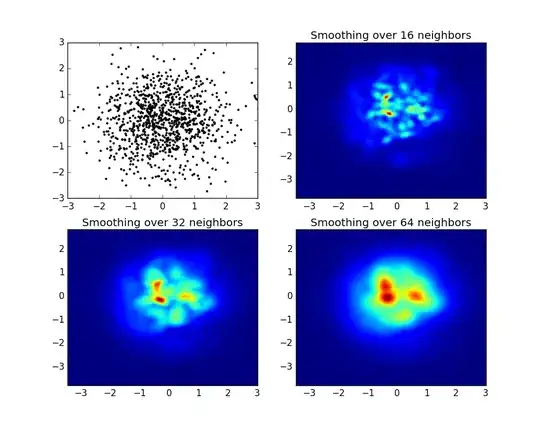
I have the following picture which is a photo of pancreatic cells 
What I would like to do is being able to get the membrane of each cell (red filament) and then do a tessellation in order to get an idea of the length of a filament. So far I have tried to use the example given on the matlab website but the result is not really good...
I = imread('picture.tiff');
I_gray = rgb2gray(I);
[~, threshold] = edge(I_gray, 'sobel');
fudgeFactor = .5;
BWs = edge(I_gray,'sobel', threshold * fudgeFactor);
se90 = strel('line', 3, 90);
se0 = strel('line', 3, 0);
BWsdil = imdilate(BWs, [se90 se0]);
I have been searching for hours other way to do it but without any satisfying result... Is there a way to do so ? Maybe an other software than matlab could be more efficient. Thank you by advance !