Atoms are too small to see with microscopes that rely on light (also due to problems such as the Abbe diffraction limit). Essentially, with a conventional microscope you cannot distinguish points that are too close.
Even the recently introduced superresolution microscopes, which can surpass this limit, are still not able to image single atoms (they can, however distinguish single molecules, with a distance of <20nm).
This is mostly due to the fact that these microscopes rely on light, which has a wavelength that is too long to "catch atoms" (pardon the non-scientific wording of this sentence).
However, several other techniques exist that allow us to "see" atoms. I would like to stress that the very nature of atoms prevents us to actually have a photo of them like we take a photo of a macroscopic object.
Various techniques exist:
High resolution electron microscopy (HRTEM), which uses the phase-shift of a wave of electrons thrown at the sample.
This is an example of an indium nanoparticle imaged with HRTEM:
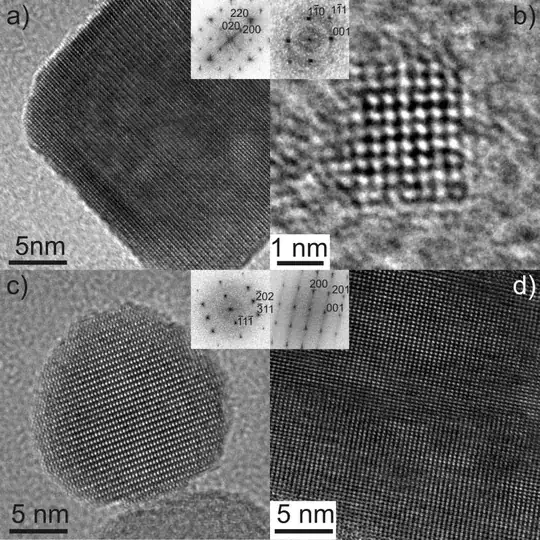 (From: "Current Research", Prof. Dr. Nicola Pinna )
(From: "Current Research", Prof. Dr. Nicola Pinna )
These are HRTEM images of a superconductor:
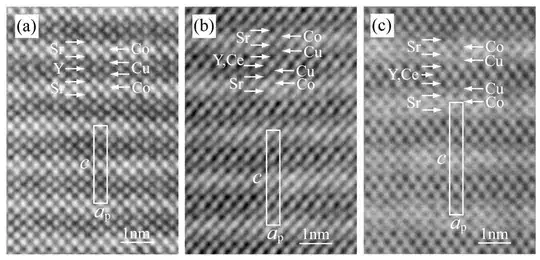 (From: "Structural order and disorder in Co-based layered cuprates CoSr2(Y,Ce)sCu2O5+2s (s = 1-3)", National Institute of Material Science )
(From: "Structural order and disorder in Co-based layered cuprates CoSr2(Y,Ce)sCu2O5+2s (s = 1-3)", National Institute of Material Science )
Another technique is X-ray crystallography.
I am not going to explain the mathematics and physics beyond it, as they are quite complex but essentially you take a crystal of the molecule you like, shine an X-ray beam on it and look at how the X-rays are scattered by the atoms in the crystal.
The diffraction pattern does not per se show the single atoms, but mathematical analysis of the diffraction spots allows for reconstruction of an electron density map and subsequently of the structure of the molecule.
Probably one of the most famous X-ray diffraction patterns is Photo 51 made by Rosalind Franklin of DNA, which was then used by the Nobel prize winners James D. Watson and Francis Crick.
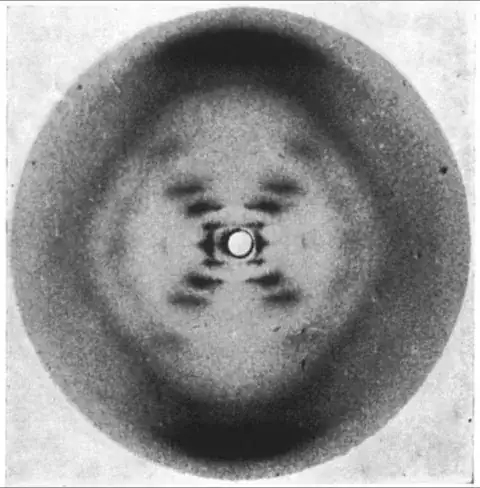
See also some published papers at this regard.
Finally another technique is the Atomic force microscope. The principle is to have a very small cantilever that can pass over the sample and gets pushed up and down due to interaction with the electron fields of atoms.
An enhanced version of AFM was developed in 2009, which allows to see atoms and bonds in a molecule.
The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy - Gross et al. - Science 2009

Pentacene molecule. Top: ball and stick model, middle: classical SFM, bottom: enhanced SFM. Scale bar 5 angstrom.
Update: Scientests have now taken a photograph of a single ytterbium atom, showing the shadow created by a laser hitting it.
 (From: "
(From: " (From: "
(From: "