The answer is NO, but the question is poorly formed and we have to interpret it to get anything like a reasonable answer
The other answers have already addressed why a simple interpretation of "one molecule away from" doesn't make much chemical sense. But if we generously interpret the intent of the question in a way that makes chemical sense as something like "margarine is made of molecules that are similar to some plastics" we can answer more precisely.
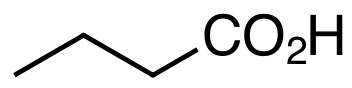
To do so we need to draw some pictures of what typical molecules plastics are made from and what margarines (and butter for that matter) are made from. To avoid excessive clutter in structures chemists draw the carbon skeleton (often omitting the letter C for carbon and trusting readers to fill in the hydrogens so each carbon node has 4 bonds in total). So we draw butanoic acid (CH3CH2CH2COH) as:
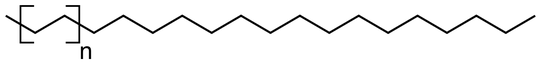
Packaging is commonly made of plastics like polyethylene which as a structure like this where n is a large number often in the thousands:
This is probably the one the question likens margarine to, but there are lots of other common plastics. PET (polyethylene terephthalate) is commonly used in packaging (especially soft drink bottles) and looks like this:

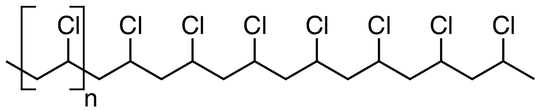
Another common plastic used in packaging, cling film and many other uses is PVC which looks like this:
Only polyethylene bears any resemblance to the constituents of margarine.
So what is margarine made from? Margarine is actually very similar to butter in that it consists of a mixture of natural oils which in turn consist of triglycerides of fatty acids. A typical margarine (see here) might contain a lot of this, for example:
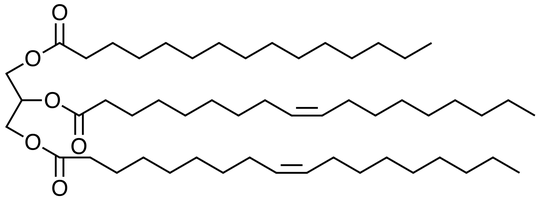
Triglycerides are compounds of glycerol and three fatty acids. Fatty acids (which, when separated from the glycerol, are the key constituents of soap) are compounds like this (this is linoleic acid with 18 carbons and two double bonds) with even numbers of carbons from 4 to about 22 in typical plant and animal oils .
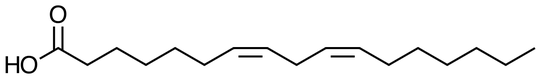
Typical plant oils are liquids at room temperature and people want their spreads to be solids, the plant oils are usually chemically altered a little to create compounds that are solid at room temperature. Since the oils containing fatty acids with multiple CC double bonds are more liquid, food companies use a process called hydrogenation to add hydrogens giving constituents that are solids at room temperature. These are the parts of the margarine that bear a superficial resemblance to polyethylene.
But there are major differences. The fatty acid groups are unlike the simple carbon chains of the polymer because they are terminated with a CO2R group which allows the digestive system to chop up the chain, two carbons at a time, to generate energy. You can't do that with polyethylene as there is no "handle" at the end of the chain for the digestive system to get to grips with. So a superficially small chemical difference makes a huge biological difference. And, of course, the chemical functionality allows the fatty acids to be moved around as the triglycerides (which are nothing like any of the plastics).
Moreover, the carbon chains in fatty acids are rarely longer than 22 carbons whereas polymer chains often have thousands of repeat units. This length alone makes typical polymers biologically inert as they are too insoluble to be digested in normal biological systems (even assuming there was a "handle" for the systems to grip to start the digestive process.)
So despite the superficial resemblance between some plastic polymers and fatty acids, they have little chemical resemblance and the claim that margarine is one molecule away from plastic is nonsense. Moreover, butter, a "natural" product differs from margarine only in having a different mix of fatty acids (it is also mostly made of triglycerides). And some of the fatty acids are essential nutrients for mammals.
Some general references:
Some information about fatty acids and triglycerides
Typical compositions of butter and margarine
Analysis of butter and margarine reference (paywalled)





