I've always kept bottles of fizzy drinks inside the fridge, but I've been told by many that doing so will result in the drink becoming flat much quicker and that I should keep bottled fizzy drinks outside the fridge. I guess I've just blindly accepted the "keep refrigerated" marker on the bottle. So, the question is, which is correct? Should they be kept outside the fridge or inside?
Asked
Active
Viewed 1.7k times
8
-
1I like vartec's answer below. It's based on science and correct; however it doesn't really answer the question. Questions are: when the bottle is full, what influences the solubility of the gas more - the pressure or temperature; likewise when the bottle has less fluid in it. Another thing to consider is the orientation of the bottle: upright the fluid at the bottom is under higher pressure than that at the top, but when (possibly) lying down in a refrigerator the pressure on the bottom of the fluid is weaker (lower depth). How much influence do these play, if any? – Paul McCabe Jun 29 '11 at 14:23
-
1@Paul: the only way pressure can build up in sealed bottle is by CO2 escaping from the water, and CO2 escaping the water is exactly what you want to prevent. – vartec Jun 30 '11 at 11:21
-
2@vartec: Unless the temperature changes and the air pressure varies with it (i.e. in a fridge) – Paul McCabe Jul 01 '11 at 15:36
-
1@Paul: fridges are not airtight, seals are perforated. Otherwise you'd have trouble opening them. – vartec Jul 01 '11 at 15:48
-
2@vartec I was referring to the air pressure varying with temperature in the sealed bottle. :) – Paul McCabe Jul 01 '11 at 21:48
-
I've noticed it goes flat quicker in the fridge. I keep mine out of the refrigerator. – Nov 12 '13 at 19:57
-
I prefer my fizzy drinks cold. So I keep them in the fridge. And I doubt that marginal differences in lifetime would compensate for that even if being warm made them last longer, which it probably doesn't. – matt_black Nov 12 '13 at 23:26
-
A sealed bottle is a closed system. So long as nothing leaks, changes in temperature aren't going to make any difference. Left alone everything will return to a steady state. The only significant factor is the temperature of the contents *at the time the bottle is opened*. – Ray Butterworth Dec 04 '19 at 17:00
1 Answers
15
Solubility of gases, including CO2 decreases with temperature.
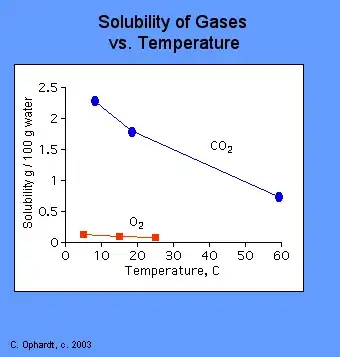
Thus, with constant pressure, warm carbonated drink will go "flat" much sooner than cooled one.
In case of sealed bottle, effect is much lesser, because solubility increases with pressure. Leaving your sealed soda bottle warm will build up pressure with CO2 escaping the water, preventing more gas from escaping the solution. This is described by Henry's law.
vartec
- 26,581
- 5
- 97
- 155
-
1Is it possible that a warming bottle (i.e. a bottle that was in the fridge, was opened, and now isn't in the fridge) might lose its carbonation faster than a steady-state (not previously refrigerated) bottle that's already warm? – ChrisW Jun 29 '11 at 12:22
-
-
@vartec - The question may be about warm glass touching cold liquid, compared with warm glass touching warm liquid. The precipitation of carbonation is complicated: e.g. it depends on the smoothness of the surface on which a bubble may form. My guess is that you're right, but I'm not sure that "standard physics/chemistry" is enough to be sure (i.e. whether your theory covers this situation, or whether it's possible that this situation introduces confounding factors (e.g. precipitation on the boundary layers) that might mitigate the standard theory). – ChrisW Jun 29 '11 at 12:40
-
@Chris: I've heard many theories, like for example that it's better to rinse glass, rather than pour into dry one. Verification of that goes far beyond basic laws of physics, and I kind of doubt if there is any serious research done on the subject. But IMO, if it would have any effect at all, it'd be relatively small comparing to effect of cold vs warm drink. – vartec Jun 29 '11 at 12:45
-
-
I concur with @ChrisW. I think this covers well the first order effects, but could there be other effects (shrinking seals, deteriorating plastic, chemical reactions with the ingredients causing poorer solubility, exposure to ethylene from rotting fruit in the crisper... I am just making junk up, to illustrate the point.) – Oddthinking Jul 01 '11 at 01:54
-
3To me, it seems more likely that our perception of "fizziness" likely comes from the **release** of CO2 rather than the amount of CO2 that's in solution. Ergo, while it may be the case that a colder glass of soda has more CO2 in solution than a warmer glass of soda, the warmer glass would likely appear to be more fizzy because its CO2 is falling out of solution, which would produce bubbles and a fizzy sensation while drinking. If this is correct, then it would probably be best to store the soda cold, but allow the bottle to sit out for 10 minutes to cause more CO2 to fall out of solution. – Michael Jul 01 '11 at 07:54