There is a lot to unpack from that site; the majority of it seems to have little basis in reality. The short answer is that you cannot permanently change the hydrogen bond angle (104.5°) in water.
EDIT: Free H+ binds to water molecules to form H30+ hyrdonium ions which do have a bond angle of 113°, these are small in number even in very strong acids and would not be more common in purified water.
What is the bond angle determined by?
Water is based on a tetrahedral structure, where 2 of the 'corners' are instead electron pairs, giving it an overall "angular" structure. It is useful to visualise how this looks in 3D compared to the 2D visualisations we may be more familiar with:
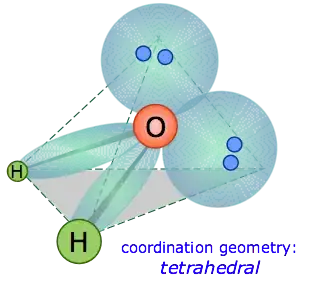
https://socratic.org/questions/what-is-the-bond-angle-in-a-water-molecule
Tetrahedral structures have a bond angle of 109.5°, however, water has a smaller bond angle due to repulsion between the two electron pairs. This can be determined both theoretically using molecular orbital simulations and empirically using x-ray crystallography.
This would ordinarily result in a tetrahedral geometry in which the
angle between electron pairs (and therefore the H-O-H bond angle) is
109.5°. However, because the two non-bonding pairs remain closer to the oxygen atom, these exert a stronger repulsion against the two
covalent bonding pairs, effectively pushing the two hydrogen atoms
closer together. The result is a distorted tetrahedral arrangement in
which the H—O—H angle is 104.5°.
https://www.chem1.com/acad/sci/aboutwater.html
What about vibration?
One of the modes of vibration of water molecules is "bend" where vibration of the atoms does indeed cause the atoms to wiggle about and the bond angle to change. However, this is not a permanent state that can be "locked", all molecules vibrate and the vibrations happen at well characterised frequencies which can be measured by spectroscopy.

https://pubs.rsc.org/en/content/articlelanding/2020/cp/c9cp07042g#!divAbstract
ETA: What about pH?
Hydrogen ions in water readily form H3O+ Hydronium ions, which, once again, do have a different bond angle (113°). However these will be vanishingly small in number and purifying water would certainly not make these more common.

