So far, this appears to be a Yes, provided one has the right tools.
I was first going to look at the chemistry involved, but figured it would be easier to just look at one of the top hits for chargers claiming to do this and examine its patent.
One such device is the Battery Xtender.
I searched for patents containing "alkaline battery recharger" and came across a few. I wanted to try to figure out which one was responsible for the "patented technology" claim in the link above. I simply searched the applicant's name and "battery xtender" and got it on the first hit: JD Pfeiffer of Quebec, Canada, owns the trademark to Battery Xtender, and his patent application was granted under US Patent #5,543,702 (LINK).
You can read the patent, which contains circuit diagrams and descriptions of preferred current supplies and test methods to determine when charging is completed.
Now... does it work? I can't be sure without testing one, but one of the requirements for a granted US Patent is utility -- it has to be useful (LINK). A charger that doens't charge is not useful. Assuming the US Patent Office is doing their job, the data provided by the applicant with the application showed that this device was useful!
You do have to jump through some hoops for this -- charging alkaline batteries requires that they not be depleted. Here's a page from their manual (LINK):
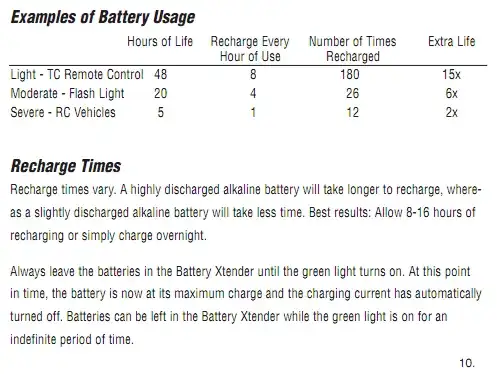
So, note that instead of using an alkaline battery until it's dead, one would cut it off about about 1/5 to 1/6 of it's normal life and recharge it. I don't know how long it takes to recharge, but this might amount to having an extra set or two of batteries if one is to keep cycling like this.
Wiki has an article HERE about this which references pulsed current for charging; the whole article is quite vague and un-referenced, though. I didn't see anything about pulsing in my read of the Pfeiffer patent.
So, with the right type of charger, one does appear to be able to recharge alkaline batteries intended for single use if recharged frequently and before draining very much.
I wish there were more available test data from this device from external users/organizations. So far, nothing like that. Here's the best I was able to find. Perhaps some independent, controlled tests/reviews will come about down the road.
