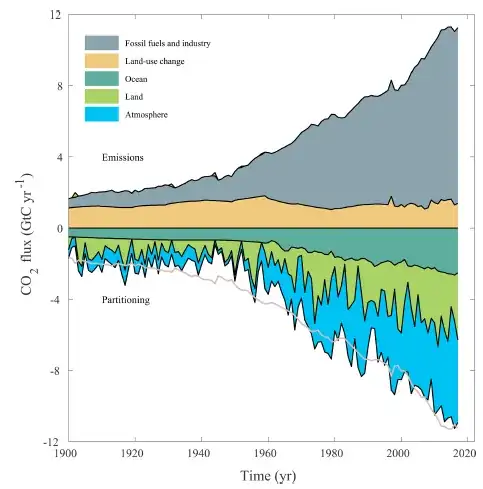
In Bill Gates' 2010 TED talk, Bill Gates: Innovating to zero! [YouTube], Gates mentions that he has
asked the top scientists on this several times: Do we really have to get down to near zero? Can't we just cut it in half or a quarter? And the answer is, until we get near to zero, the temperature will continue to rise. And so that's a big challenge. It's very different than saying, "We're a twelve-foot-high truck trying to get under a ten-foot bridge, and we can just sort of squeeze under." This is something that has to get to zero.
[emphasis mine]
This is in relation to the emissions that we are producing and the need for these levels to go right down to near zero. This intrigues me as it's a very substantial claim.
Do the Climate Emissions need to go right down to near zero to stop the temperatures rising?