Part of the confusion is in language usage. Most salts are bleached. In the sense that "bleaching" means 'to make lighter' or 'to whiten'. "Bleaching" is not restricted to 'using a technical/chemical bleaching agent'. Any process used to make the product more pale or more white is 'bleaching'.
(Cf — Gerhard Eisenbrand & Peter Schreier: "RÖMPP Lexikon Lebensmittelchemie", Thieme: Göttingen22006. (p141–142)")
In historical documents we see numerous references to 'bleached salt'.
It is all about (perhaps local) legal definitions.
Making the question in claim a tautology:
refined salt is 'bleached salt'. Not all kitchen salts is refined though.
Only salt sold as found is 'unbleached.'
Since most types of rock salt are not pure enough to be sold as food use salt, those are all made 'lighter'. But in all probability not with a chemical we commonly call 'bleach'. If we read somehwere "not refined, not purified, and not bleached" in relation to salts, we read a triple plenasm (in the better cases.
This goes far enough that the European Union publishes for example the following:
‘SEL DE SALIES-DE-BÉARN’
EU No: FR-PGI-0005-01311 – 03.02.2015
Publication of an application pursuant to Article 50(2)(a) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council on quality schemes for agricultural products and foodstuffs
(2016/C 53/08)
Official Journal of the European Union 12.2.2016
3.2. Description of the product to which the name in 1 applies
‘Sel de Salies-de-Béarn’ is an edible salt. It comes in the form of fine salt or coarse salt. It is neither refined nor
washed after harvesting, and is produced without the addition of technological additives.
A comparative study by D. Cussey-Geisler et M.H. Grimaldi (‘Caractérisation du sel utilisé pour la salaison du Jam bon de Bayonne (Characterisation of the salt used for the salting of Bayonne ham)’, UPPA, July 1989) of several salts from different sources concluded that, from a geochemical point of view, ‘Sel de Salies-de-Béarn’ has a wide variety of trace elements, in particular calcium, potassium, magnesium, and different sulphates, that are characteris tic of the Triassic evaporites.
These specific characteristics give ‘Sel de Salies-de-Béarn’ a strong reputation.
Causal link
The link to the origin of ‘Sel de Salies-de-Béarn’ is based on its quality and reputation. The combination of natural factors and ancient know-how lead to the creation of this unique product.
The geographic area’s spring water became very rich in minerals upon contact with the geological layers. The trace elements present in this spring water can be found in the fluid inclusions of crystals of ‘Sel de Salies-de-Béarn’.
The ancient know-how respects the intrinsic qualities of the spring water and makes crystallisation possible, with out any human intervention other than the heating of water, giving ‘Sel de Salies-de-Béarn’ all of its richness.
This same know-how helps to preserve the natural colour of ‘Sel de Salies-de-Béarn’, because special attention is paid to the storage and packaging stages, in particular by preventing oxidation and increases in moisture content.
Lastly, by keeping handling to a minimum it is possible to preserve the characteristic structure of ‘Sel de Salies-de- Béarn’ in the form of clusters of inverted pyramids.
Numerous historical accounts give testimony to the reputation of ‘Sel de Salies-de-Béarn’, such as ‘Mélanges historicques critiques de physique, de littérature et de poésie (Assorted critical reviews of physics, literature, and poetry)’ (1768), in which the Marquis d’Orbessan described the production of salt at Salies-de-Béarn. He concluded his story with the following observation: ‘There is nobody who does not know that one must bleach ordinary salt to remove its briny flavour and make it fit to be served on tables. Salies salt does not need to be treated in this way.’
Today, salt still has a central role in the social and cultural life of the town of Salies-de-Béarn, which promotes salt through dedicated associations, artistic works, and cultural activities. Thus, every year for more than 30 years, the ‘Jurade du sel’ association has organised the famous ‘Hesta de la sau (salt festival)’. This event brings together more than 10 000 people every year. All year around, many tourists come to visit ‘the city of salt’.
‘Sel de Salies-de-Béarn’ is used to produce well-known products, such as the ‘Jambon de Bayonne’ PGI, ‘Ossau-Iraty’ PDO ewe’s milk cheese, and the ‘Canard à foie gras du Sud-Ouest’ PGI (Chalosse, Gascogne, Gers, Landes, Périgord, Quercy).
Many chefs and chocolate-makers also use ‘Sel de Salies-de-Béarn’.
Using chemical bleaching agents to suppress the optical appearance of (dis-)colourants in salt is not allowed under current European law. (While a refined salt shouldn't need this anyway—pure NaCl doesn't produce much colour…)
Bleaching agents that are allowed, in other foodstuffs, are regulated in laws like for example
"Verordnung über die Verwendung von Extraktionslösungsmitteln und anderen technischen Hilfsstoffen bei der Herstellung von Lebensmitteln (Technische Hilfsstoff-Verordnung vom 8. November 1991 (BGBl. I S. 2100), die durch Artikel 1 der Verordnung vom 27. September 2017 (BGBl. I S. 3518) geändert worden ist" (THV))(PDF).
This law specifies that for foods only sodium hypochlorite, potassium permanganate and hydrogen peroxide (NaOCl, H2O2, KMnO4) are allowed. Allowed to remain in the food as sold, that is.
The funny thing about this law is that strictly it would allow non-declaration of for example sodium hypochlorite to be used during salt raffination, as that will have done what it should before it is sold. Plus it would and should also degrade from sodium hypochlorite to sodium chloride anyway. And sodium chloride is ordinary salt. This is a hypothetical. Hypochlorite usually doesn't bleach what in most salt deposits causes the colouration. If it is that dirty of a salt, other methods are preferable.
The Bavarian Ministry for Environmental and Consumer Protection clarifies that even this kind of processing is not allowed under German law for one specific form of salt:
Stone Salt (rock salt, here labeled Steinsalz)
The large lumps of salt are coarsely crushed, finely ground and packed. As table salt, rock salt must be very pure. This is true if the salt consists of at least 97 percent sodium chloride. Pure table salt is inherently white. It must not be bleached. If the salt dome contains geological conclusions such as copper or clay, the rock salt is processed into evaporated salt to remove these hardly soluble compounds.
Evaporated salt (Siedesalz)
The basis for evaporated salt is the brine (salt solution). Solutions rich in common salt arise naturally and artificially when fresh water is introduced into salt domes. After pumping up, evaporators concentrate the brine in the salt domes. In the process, other minerals are separated and the pure table salt crystallises out. Most of the pure table salts are evaporated salts. They have a strong taste of salt and meet the quality requirements for a table salt with a high degree of purity.
— Jutta Kamensky - VerbraucherService Bayern: "Salz", Bayerisches Staatsministerium für Umwelt und Verbraucherschutz, Stand: 31.07.2018.
We see that, under the working definitions of this ministry, mined stone salt is unbleached (here: minimally processed) and white. If the mined salt is not white enough, it is treated to remove non-white components, that is whitened, or bleached, and has to be sold under a different label. This ministry confirms that most salts available on the market it controls are bleached (in this way).
A German organic food producers association follows a similar definition: "Ursalz" as allowed by them is >97% NaCl, sold as mined, including all impurities as they occur. It is "unbleached", as they say.
The international standard Codex Alimentarius basically just specifies a sodium chloride content of above 97%. (Standard For Food Grade Salt
Codex Stan 150-1985. PDF)
The Alpine salt saline in Reichenhall uses water to extract a solution made from large 'stone salt' deposits, then uses mechanical means to filter it, then evaporates the water with heat. That removes the clay like impurities, whithens/bleaches the product and despite being from 'stone salt', the products are then sold as 'evaporated salt'.
The salt mining association VKS has a brochure explaining some processes used.

If this mechanically mined salt is >97% NaCl, just broken down and otherwise chemically/biologically uncontaminated, it can be sold as unrefined/unbleached 'stone salt'. It may be really white, or slightly off:


If this stone salt is not pure enough, it is refined. In this case bleached, by making a 26.4% saltwater solution, then using filters, centrifuges, sedimentation/decantation, and thus a whitening/bleaching process, perhaps just the heating:
halites from Kłodawa Salt Mine. UV–vis spectroscopy was used for detection of colour centres in blue halite single crystals and their bleaching under heating. (src)
which is also used for all water-based mining processes:
The brine purification process
Saturated brine is the starting product for the production of evaporated salt, for soda production and for the electrolytic extraction of chlorine and caustic soda. The brine contains as by-product parts of calcium, magnesium and sulphate ions and is subjected to chemical brine purification before further processing.
The removal of the dissolved calcium and magnesium ions is mainly achieved by treating the raw brine with milk of lime and soda as well as flue gas (CO2) or also with caustic soda and soda.
— Verband der Kali. und Salzindustrie eV: "Salz aus Deutschland Gewinnung und Verwendung" (PDF)
Meaning that for example "'Himalayan' salt" falls almost regularly short of that definition used by Codex Alimentarius, but is indeed unbleached. Mined, broken up, packaged, shipped, price increased once reaching Western shores, then sold. 'Almost' as some analysis showed 94–96% NaCl content, but at least if the 'Himalayan' salt is just repackaged Polish stone salt it has 97–98% NaCl.(References)
— Salt Production | How It’s Made
How another producers explains his processes:

Alternatively, this explanation sums it up:
The Table Salt of Today
After the salt is harvested, it typically goes through a bleaching process to remove any impurities. Bleaching in turn strips away all of the natural trace minerals.
After clearing up language usage, these are the actual processes used:
For refining rock salt
- Selective Comminution and Sieving
- Separation in a Throw Parabola
- Manual Sorting
- Optical Sorting
- Heavy-Medium Separation
- Flotation
- Electrostatic Separation
- Magnetic Separation
- Thermoadhesive Process
All of the above processes increase the NaCl concentration without a chemical bleach. (list from Ullmann) Considering that in mines like on Sicily the NaCl concentration already exceeds 99%, this can be marketed as is. The impurities removed by the above processes are only needed for less pure deposits, like in South Germany, or when introduced by the mining process itself (eg with explosions).
Evaporated salt / vacuum salt
If it is not broken up from the rock and packaged then, water is involved. Either from getting it out via a brine solution underground, or concentrating sea water.
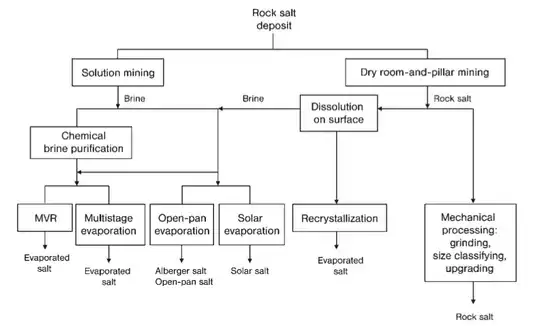
As the claim asks for 'bleach' in the sense of "any chemicals used", the chemicals involved in the purification of brines, if used at all, are:
The most common and most problematic impurities in crude salt are the sulfates, chlorides, and, to some extent, the carbonates of calcium and magnesium, as well as the triple salt poly- halite (K2SO4 2 CaSO4 MgSO4 2 H2O). In addition the water which is used for dissolving rock salt contributes to the content of impurities in the crude brine. Most of the magnesium com- pounds are very soluble in crude brine. Further- more, the crude brine is saturated with calcium sulfate in many cases. Sodium chloride brine significantly enhances the solubility of calcium sulfate [54]. This effect is called "salting-in".
The principal impurities of crude brine are therefore calcium, magnesium, and sulfate ions, but also strontium ions, all of which take part in scale formation. Potassium ions are irrelevant for the formation of incrustations in the evaporation equipment due to their high solubility. They only play a role as impurities in the final high-purity product
The resulting brine is usually repeatedly fed into low pressure evaporation reactors, with each stage decanting the sludge of unwanted impurities.
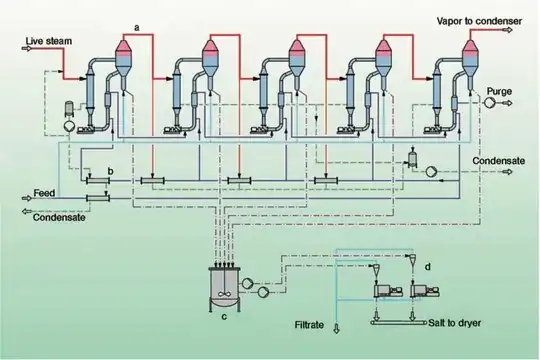
If a higher purity is desired, Re-Crystallisation processes come in economically.
The recrystallization process was first introduced in the salt industry in 1951 by International Salt as the Richards process and by Salins du Midi as the Pompe a Sel process.
And since 1994 nano-filtration is available.
— Gisbert Westphal, Gerhard Kristen, Wilhelm Wegener, Peter Ambatiello, Helmut Geyer, Bernard Epron, Christian Bonal, Georg Steinhauser & Franz Götzfried: "Sodium Chloride", Ullmann's Encyclopedia of Industrial Chemistry, 2012.
These processes bleach slightly non-white to even black appearing salts. Chlorine, is made from these salts, but not used to purify it. Sulphuric acid would form sulphate ions which would be subject to removal anyway. No kitchen, cleaning or washing machine 'bleach' makes any sense in those processes.
Another explanation for brine evaporation technique, as used in the UK for 'white salt'.
In Mexico sea salt is simply concentrated by surface evaporations to a purit of 98.54% then simply washed to achieve 99.7%, in Port Hedland, Australia a similar process achieves a washed quality of 99.8% (Ullman's) In New Zealand's Dominion Salt site at Lake Grassmere food grade salt is also obtained via these methods, concentration, precipitation, decanting, and finally washing. Even higher purity requires then a vacuum plant as well.
In an old summary:
Natural salts are often processed to remove some of their impurities before sale. All food-grade and certain chemical applications require this. In the chlor-alkali industry, it is necessary at some point to convert crude salt as mined or produced into a material suitable for use in electrolysis. Whether this occurs at the salt producer's site or in the chlor-alkali plant is fundamentally irrelevant. For economic or technical reasons, the salt supplier often undertakes a certain amount of upgrading. The most important processes are salt washing, brine evaporation, and salt recrystallization.
Food-grade salt, for instance, is almost exclusively the product of evaporation of NaCl brine.
— Thomas F. O'Brien, Tilak V. Bommaraju & Fumio Hine: "Handbook of Chlor-Alkali Technology. Volume I: Fundamentals; Volume II: Brine Treatment and Cell Operation", Ch 7 "Brine Preparation and Treatment", p478, Springer: New York, 2005.
And this is how judges in India see that matter of claiming salt to contain bleach:
The Indian Salt Manufacturers Association (ISMA) has obtained an exparte ad interim injunction against Puro Wellness Pvt Ltd from advertising and promoting their brand Puro Salt through an advertisement that denigrates and disparages the white salt available in the market. ISMA had filed a Civil suit before the City Civil Court Ahmedabad.
Puro salt is being endorsed by actor Anil Kapoor. The said advertisement has baselessly questioned the purity and quality of white salt available in market and compared white salt with bleach. Further, the advertisement conveys the message that the white salt is manufactured in chemical factory and is bleached by bleaching agents, the petition stated.
ISMA has contended before the court that the said advertisement commercial is false and baseless and creates a wrong impression in the minds of the consumers who will feel that white salt isn't good for health.
Jatin Trivedi, ISMA’s advocate stated while Puro Wellness may have said their pink salt is good for health, but cannot assert through advertisement that white salt in the market is bad and unhealthy.
— Shramana Ganguly: "Indian Salt Manufacturers Association get an exparte ad interim injunction against Puro Salt", IndiaTimes, Mar 21, 2018.






