Younes Shabany, professor in the Mechanical Engineering department at San Jose State University, writes in Heat Transfer: Thermal Management of Electronics:
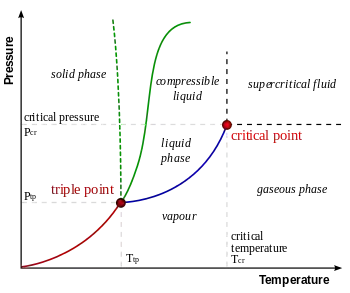
For example, boiling temperature of water is a function of its pressure; water always boils at 100°C if the pressure is 101.42 kPa
Hasok Chang, professor at the University of Cambridge for History and Philosophy of Science, writes in The Myth of the Boiling Point:
We all learn at school that pure water always boils at 100°C (212°F), under normal atmospheric pressure. Like surprisingly many things that "everybody knows", this is a myth. We ought to stop perpetuating this myth in schools and universities and in everyday life: not only is it incorrect, but it also conveys misleading ideas about the nature of scientific knowledge. And unlike some other myths, it does not serve sufficiently useful functions.
There are actually all sorts of variations in the boiling temperature of water. For example, there are differences of several degrees depending on the material of the container in which the boiling takes place. And removing dissolved air from water can easily raise its boiling temperature by about 10 degrees centigrade.
Is Hasok Chang account, that the standard ideas taught about the boiling point of water are flawed, true?