I knew this would work theoretically, but I looked for people actually performing the experiment empirically.
To my surprise, I found a carefully performed experiment in a 2014 blog article that concluded:
BUSTED! Depending on how you wrap the paper towel it will either have no effect or slow down the cooling of your favorite drink.
Here is the killer diagram (referring to glasses, not bottles):
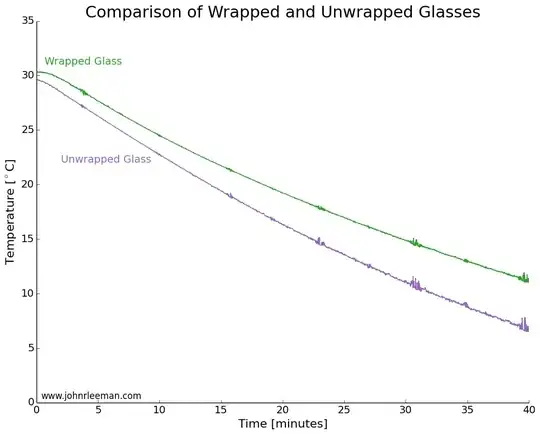
He shows that for pint glasses, the unwrapped version cooled faster, but for bottles there was no major difference.
However, the comments on the blog include people whose experiments agreed and disagreed, including a link to Physics.SE: Does wrapping a wet paper towel around a glass bottle really speed up the cooling process? where one person's experiment revealed the direct opposite:
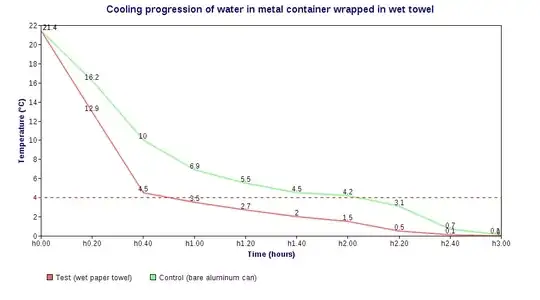
[...]
So yes, the wet paper towel trick does seem to work quite nicely. I'd expect it to work even better if one were to use a ventilated freezer (faster heat exchange) and smaller containers (greater surface/volume ratio).
Obviously these are amateur non-peer-reviewed results, but in the absence of more serious research (I did look, but came up empty-handed), I am forced to say "It is still unclear."
(Meanwhile, in my head, I switch back and forth in my views. Maybe more contact with the cold walls of the refrigerator, by placing the bottles horizontally, would improve the effect of the water's thermal conductivity, but without that, it is just another layer of insulation between the air and the drink?)

