The classic old method: manure, especially on your case, where you have a nearly neutral soil. But in general fertilizers acidify the soil, so often this is enough for vegetable garden (one fertilize near seedling). Not doing lawn scarifier helps (also this remove acidity, which is bad for lawn in a acid soil, but not so bad on normal soils). On flower garden: some plants/tree help to acidify soil, so with a good placement of element, you can have a acid corner. Peat-like substances are (should be) also acidifier.
Normally plants usually tolerate acid soil (but not extreme), but only plants evolved to tolerate Calcium can live in a non-acid soil: roots absorb nutrients, but if there is much calcium, acid plants absorb calcium and not the other needed substances.
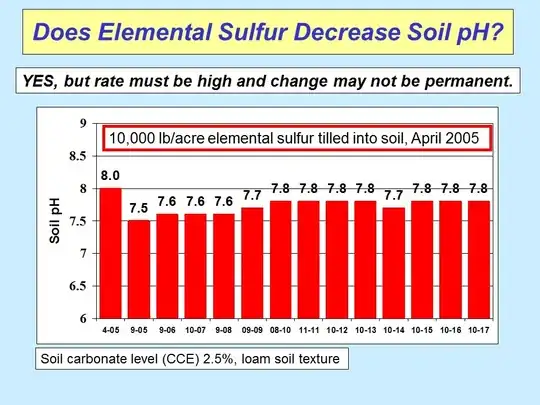
About your product: "Sulphur" usually (and unfortunately) is just a nametag. Also that should be a sulphur salt/component.
The possible problem: the soil is huge, so nutrients moves, and basic soil will neutralize your acidifier, but the real problem is the watering. Usually water is also not neutral if your soil is not neutral. So you need to acidify periodically. Microorganism could not like many changes on acidity, so soil could become poorer. But this is often a problem on the inverse step: there is already acid parts on soil (see decomposition of leaves), so also acidophile organism. Soil pollution is also a problem, because acidifier could go into groundwater. But this is usually a problem with huge fields (and fertilizing at the same instant such large area).
So: check acidity of your water, and the structure of your soil. If water is not so basic, it is ok, else consider to collect rain water (before soil will increase the pH). If soil is light and sandy, your acidification will fail, and basic ions will enter so easily (so you need a waterproof "wall" inside the soil). On the other cases (more common): if you are building new gardening (in deep), you can uses several (and slow) acidifier on the base of soil. In any case, try with more natural methods and limited near root of the vegetables you need to acidify. Sulphur is not so bad (it is a common natural element), just it could wash away and cause some problem on other places, and drastic pH changes kill the soil, but nature will recover quickly.