When you cook with wine or spirits, when does the alcohol cook away? Obviously high temperatures will do it, but how low of temperatures will work? Also, does it vary by the type of alcohol?
-
The process is called reduction. I'll let someone else answer the main question, as I tend to do it by taste... – Aaronut Jul 28 '10 at 16:05
-
Aaronut's comment above is in response to my question which was closed as a dupe and merged with this: http://cooking.stackexchange.com/questions/3584/how-long-does-it-take-for-alchohol-to-boil-out-of-a-dish – squillman Sep 16 '10 at 01:58
7 Answers
You will never fully cook away alcohol, only reduce the amount. See Alcohol retention in food preparation, or for the quick table, see wikipedia.
They covered this on an episode of America's Test Kitchen, and concluded that surface area matters -- a wider vessel would cook off more alcohol; it wasn't just a function of time.
- 78,818
- 17
- 154
- 448
-
Could you briefly summarise the contents of the links? As it stands, the answer doesn’t say anything much on its own, and if the links were to break it would be mostly useless. — In fact that’s already the case: the first link is a content-less placeholder (I realise the answer is almost a decade old but this thread continues to be cited). – Konrad Rudolph Jan 03 '20 at 18:42
-
1The summary : alcohol is never fully cooked away, only reduced. The reduction is a function of time, technique used (eg, burning it off first, lid on vs. off, etc.), and the surface area of the vessel. But there's no point in summarizing the first two, as the third link says that there's a third variable that they didn't take into account. And that last one is just a summary of an episode of America's Test Kitchen, which although it gives some mention of their tests to show what can change things it's not exhaustive or a nice matrix that people can use to determine how much alcohol is left. – Joe Jan 06 '20 at 15:58
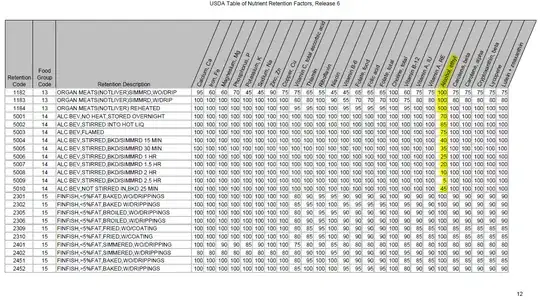
A study conducted by the US Department of Agriculture’s Nutrient Data Laboratory calculated the percentage of alcohol remaining in a dish based on various cooking methods. The results are as follows:
Preparation Method and Percent of Alcohol Retained
- alcohol added to boiling liquid & removed from heat: 85%
- alcohol flamed: 75%
- no heat, stored overnight: 70%
- baked, 25 minutes, alcohol not stirred into mixture: 45%
baked/simmered, alcohol stirred into mixture:
- 15 minutes - 40%
- 30 minutes - 35%
- 1 hour - 25%
- 1.5 hours - 20%
- 2 hours - 10%
- 2.5 hours - 5%
These data come from the USDA Table of Nutrient Retention Factors Release 6 [PDF]. The information is in the table on page 12 of the document (page 14 of the PDF file). In case that link goes dead (sorry that the screenshot is somewhat small).
-
-
2@CyberneticTwerkGuruOrc The source is [USDA Table of Nutrient Retention Factors Release 6](http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/retn/retn06.pdf) [PDF]. See p14, for example. Alcohol is listed near the right-hand side of the table. – Michael A Dec 30 '15 at 23:01
-
5This answer currently misrepresents the source in a way that may mislead readers. The retention factor is not a true "percentage of alcohol retained" but is normalized against the mass of the dish, a value that changes as it is cooked and moisture (including but not limited to alcohol) evaporates. Since the mass of the dish decreases, **the actual "percentage of alcohol retained" is lower than the retention factor implies**. – Air Jan 29 '18 at 18:43
-
2Strangely, this chart omits any methods that involve constant boiling. That method *would* remove most of the alcohol; that's how liquor is distilled. Or perhaps *not* strangely, as there's no point reporting instances where negligible alcohol is retained. – Ray Butterworth Jan 02 '20 at 16:47
If you add alcohol, some alcohol will remain indefinitely (or at least as long as the food is not a lump of smoking carbon). The proportion of alcohol to water-based liquids will shrink over time, however. (I'm assuming heat here: if there is no heat, or high pressure, then the proportion will remain stable for quite a while).
Alcohol evaporates at about three times rate of water (or to be more precise, the latent heat of evaporation for Ethanol is at 846(kJ/kg) vs water which is at 2257(kJ/kg)) but this relation doesn't hold for the proportion that will be found in your food due to atmospheric pressure and air saturation and exposed surface area...It's actually extraordinarily difficult to work out.
Roughly speaking, though, if you reduce your liquid volume you're burning off alcohol at a higher rate than water. So things that are reduced substantially will have proportionately less alcohol in them, than things that are not (all other factors being equal).
- 13,216
- 36
- 56
-
1Nice. +1. Alcohol and water, due to their chemical properties create an azeotrope and you cannot remove it all. – MarsJarsGuitars-n-Chars Feb 15 '18 at 02:10
Typically when you're "cooking" with alcohol it's as a sauce or a glaze, both of which require fairly high temperatures and would typically be done in a pan on the stove or in the oven.
As you go lower with the temperature it's going to become more of a mixed result. Some amount of the alcohol (say, the part closest to the heat source) will burn off, but some will remain.
Without more specific information about what you're trying to cook and/or accomplish it's hard to say what will work for your situation.
- 2,220
- 2
- 19
- 21
According to Wikipedia ethanol (which is the alcohol in wine or spirits) boils at 78.4 °C.
Assuming that the ethanol hasn't made a chemical connection with something in the alcohol or food, cooking at 78.4 °C for a 'sufficient' period of time, should remove any trace of ethanol.
- 901
- 6
- 15
-
1Well.. if it has made a chemical reaction, it is not alcohol anymore :) – txwikinger Jul 11 '10 at 21:08
-
@txwikinger: Not really, depends on the chemistry. Trivial counter example: if you put salt it water, it very slightly raises the water's boiling point. But its still salt and water. Now, water and ClF₃, that'd be a very different story :-P – derobert Jul 11 '10 at 21:17
-
2
-
3It *has* made a reaction -- it's an azeotrope : http://en.wikipedia.org/wiki/Azeotrope – Joe Jul 11 '10 at 21:40
-
1Those examples seem more like the effects of mixtures on physical properties than results of chemical reactions. Water and ClF3 sounds fun though :) – Kryptic Jul 12 '10 at 03:15
-
-
@derobert: Mixing water and salt does not seem to fall in any of the chemical reactions defined in http://en.wikipedia.org/wiki/Chemical_reaction – txwikinger Jul 12 '10 at 18:28
-
1@Joe: Azeotropes aren't formed by chemical reaction. They're simply mixtures in which the components boil at the same proportional rate. (And alcoholic drinks aren't necessarily azeotropes, far as I know; the alcohol may indeed boil off faster than the water, just not enough to actually matter for cooking purposes.) – Cascabel Nov 20 '10 at 19:09
-
12There's a lot of side discussion here, but the one thing that should be taken away from it: the answer is incorrect. The properties of mixtures are not simply the union of the properties of their components. The alcohol and water *both* boil, with a boiling point somewhere between that of alcohol and water, and the alcohol boils away faster, but it takes quite a long time to remove most of the alcohol, and you'll remove a lot of water too. – Cascabel Dec 24 '11 at 13:25
It is important to note that the table accompanying the USDA Table of Nutrient Retention Factors Release 6 December 2007 lists a amazing number of foods--all have 100% Ethanol retention levels for the listed food item and cooking method.
Only ALC Beverages listed and simmered as described on page 12 of the table (items with Retention Codes 5001 through 5010) have the decreasing retention percentages listed by BobMcGee and merl.
I had long assumed sufficient heat of sautéing, frying or baking removed most if not all ethanol. I am astonished to discover otherwise.
-
2Only the items with retention codes 5001 through 5010 have any significant alcohol at all. 100% of 0 is 0. http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/retn/retn06.pdf – Jolenealaska Jan 04 '15 at 10:01
If the sauce/soup/etc is above the boiling point of ethanol (about 173 F or 78.4 °C) the ethanol will boil out almost immediately. It can't remain in solution at temps above the point where it becomes a gas.
The other flavors, sugars and spices and all, will remain. Ethanol can't remain a liquid at 212 F or 100 C - physically not possible.
I don't care what Wiki says in this case. Moments after passing 173 degrees, all the ethanol evaporates from the solution, it has to.
- 266
- 1
- 2
-
4-1, as this is quite incorrect, and you know it from reading several sources, and yet still argue something different, rather than looking up why those sources say what they do. See merl's post, and Joe's post, which are informed by proper chemistry. Substances do not instantly disappear when they reach the boiling point; there is quite a lot of energy required to transition from a liquid and gas. – BobMcGee Jul 06 '11 at 18:33
-
6The boiling point of a solution is different from the boiling point of the single components of the solution. If the boiling point of the water is 212 F, and 173 F is the boiling point of the ethanol, the boiling point of a solution of ethanol and water is between 173 F and 212 F; it will be 212 F is the solution doesn't contain ethanol, and 173 F if the solution doesn't contain water. – apaderno Sep 13 '11 at 07:33
-
-
1Following this bizarre train of thought... moments after passing 100°C all the water will instantly boil off too - Huzzah! We've invented instant dried foods. – Tetsujin Dec 04 '18 at 16:25
-
1Do they give out a badge for the worst answers of all time? “I don’t care what Wiki says in this case...” is a great closer though more accurately written “I don’t care about facts in this case...” —I think this answer reached its boiling point long before it was even posted as it’s clear all logical reasoning evaporated by the time it showed up on Stack Exchange – Matthew Erwin Mar 05 '20 at 09:03