Currently I use the "shake" method of carbonation to carbonate beverages. You pressurize the bottle with CO2 at about 35 PSI then shake it vigorously for about 30 seconds and it is fully carbonated.
Recently I have discovered that beer makers use a "diffuser" which is a cylinder with microscopic holes in it to inject CO2 into their liquid (beer) and carbonate it. Will a diffuser work as well as shaking and fully saturate the liquid?
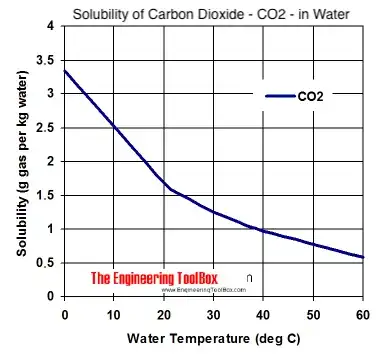
One of my concerns is that some beer manuals describe carbonation as taking hours or days. I want to carbonate a bottle in like 5 seconds rather than the 30 seconds it is currently taking me.
Typically I am carbonating one to two liters of water/syrup at a time.
Concerning Soda Stream: the problem with something like Soda Stream is two fold. First of all, their CO2 canisters are small and expensive. The other is that the momentary injection type system they use (which I could duplicate if I wanted) can only get the pressure up to about 20 PSI which is way below saturation. With my system I can easily get fully saturated 35-40 PSI, which is what a soda can or fountain produces.