Starch is extensively well-researched in food science, so the short answer is yes, there is an authoritative source; there are a myriad of authoritative sources.
The caveat (there's always a caveat) is that food scientists are doing controlled experiments using far more sophisticated methods than pasta and a boiling pot, and they tend to be primarily interested in more theoretical questions than this, because there are an absurd number of variables at work in your own kitchen:
- Type and quantity of pasta;
- Purity and quantity of the water;
- Type, purity, and quantity of salt;
- Water temperature;
- Cooking time;
- And so on...
So, really, to a scientist, the question "does adding salt to pasta water reduce sticking?" is bordering on ridiculous. The answer is, as the answer often is, it depends.
The following is taken from Starch: Chemistry and Technology, an academic tome that is all about - you guessed it - starch.
Effects of salts on gelatinization are more complex than those of nonionic solutes, as they are solute-specific and concentration-dependent. For most electrolytes, an increase in gelatinization temperature, Tm, is found at low salt concentrations. At high salt concentrations, Tm decreases and can even drop below the initial value.
[...] In general, the effect of neutral salts on starch gelatinization follows the order of the classical Hofmeister (lyotropic) series, particularly in the case of anions. [...but] cations of different chloride salts exhibit a more complicated behavior as evidenced with polarizing microscopy.
(Source)
In plain English: Adding a little salt might reduce the gelation and thus sticking. Adding a lot of salt might increase gelation and sticking. The definition of "a little" and "a lot" depends on how much and what kinds of starch are in the pasta, what else might be in the pasta (some already have salt!), how dispersed the starch already is in the water, what kind of salt you're using... you get the idea.
Incidentally, here's the Hofmeister series that the above refers to:
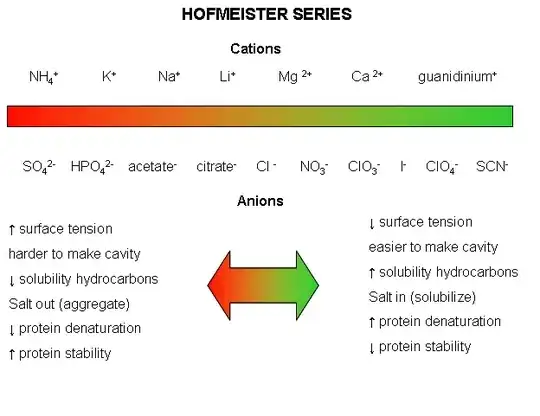
(Source)
Table salt is sodium chloride - NaCl. You'll notice that Na+ is about halfway to the "salt out" side and Cl- is right smack in the middle. So, even with my limited understanding of all the mechanics at work, it does stand to reason that the effects of having both would be unpredictable. It's easier to predict the effects when you have something like calcium perchlorate, i.e. Ca(ClO4)2, where both the anions and cations are on the "salt in" side. Obviously, those kinds of salts are not in your average home kitchen and I doubt that they're even used in commercial food plants.
...although there are other well-known culinary salts other than sodium chloride, including ammonium chloride AKA salmiac, the (in)famous "salty liquorice" salt. That is technically still "salt". Which salt you choose will of course have wildly different effects on chemical properties including starch interaction.
So hopefully this helps explain some of the wildly opposing anecdotal reports you've read. There is no simple answer because your kitchen is not controlled conditions. However, there is some truth to the statement that salt inhibits starch gelation. It might have that effect, at the right concentration and with the right set of ingredients.