I've found a lot of articles that say spinach contains more iron when boiled. However, I also came across someone saying the iron content would decrease as the boiling time increases. I was wondering why this is, if someone could answer, that would be great!
1 Answers
So, it turns out people have looked at this, and we can answer with science.
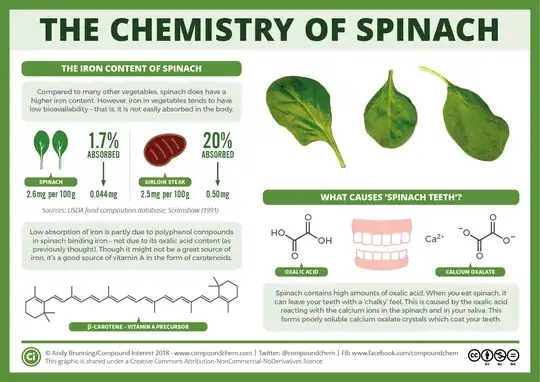
Basically, while the iron content in spinach is high relative to many other vegetables, it isn't very bio-available (i.e. not readily taken up by our bodies). This is because of some compounds found in plants, called polyphenolics. These prevent absorption of the iron by our bodies. So, while iron is at around 2 mg/100g (0.07 oz/3.5 oz) in spinach, the amount we take up is only 1.7% of that amount; 0.044 mg/100g (0.00155 oz/3.5 oz).
There's a handy infographic at compoundchem.com, which shows this:
Now, you didn't ask about that, but to make the answer more useful for other people looking on the same topic, I've included this for background. So, onto the cooking; what effect does that have.
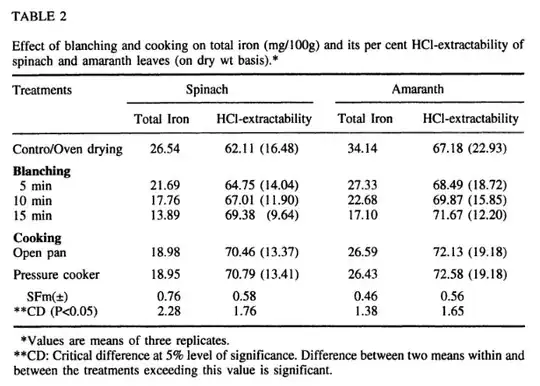
It turns out that in the paper1 (I've linked it here, it may be paywalled and is a PDF, abstract is here), iron is more available after cooking, as you can see from table 2 in the linked paper, where the extractable iron goes up after cooking:
I haven't been able to find out about whether this actually increases the bioavailability of the iron or not (I suspect not), but there is certainly more extractable iron in cooked spinach than uncooked.
1: Yadav SK, Sehgal S. Effect of domestic processing and cooking methods on total, hcl extractable iron and in vitro availability of iron in spinach and amaranth leaves. Nutr Health. 2002;16(2):113-20. doi: 10.1177/026010600201600205. PMID: 12102364.
- 12,979
- 21
- 49